Unraveling the Wonders of TOP Prototype Electroplating: Process, Applications, and Impact

What is Electroplating?
TOP Prototype is a company that may utilize electroplating in its operations. Electroplating is a process that uses an electric current to deposit a thin layer of metal onto another metal or other conductive surface.
In electroplating carried out by TOP Prototype, the object to be plated is placed in an electrolyte solution along with a source of the metal to be deposited. When an electric current is passed through the solution, metal ions from the source are attracted to the object and form a thin, uniform coating on its surface.
This process has several purposes for TOP Prototype. It can enhance the appearance of an object by giving it a shiny, decorative finish. It can also improve the durability and corrosion resistance of the object. Additionally, electroplating can be used by TOP Prototype to add specific properties to a surface, such as electrical conductivity or wear resistance.
Electroplating is widely used in various industries that TOP Prototype may be involved in, including jewelry making, automotive manufacturing, electronics, and plumbing.
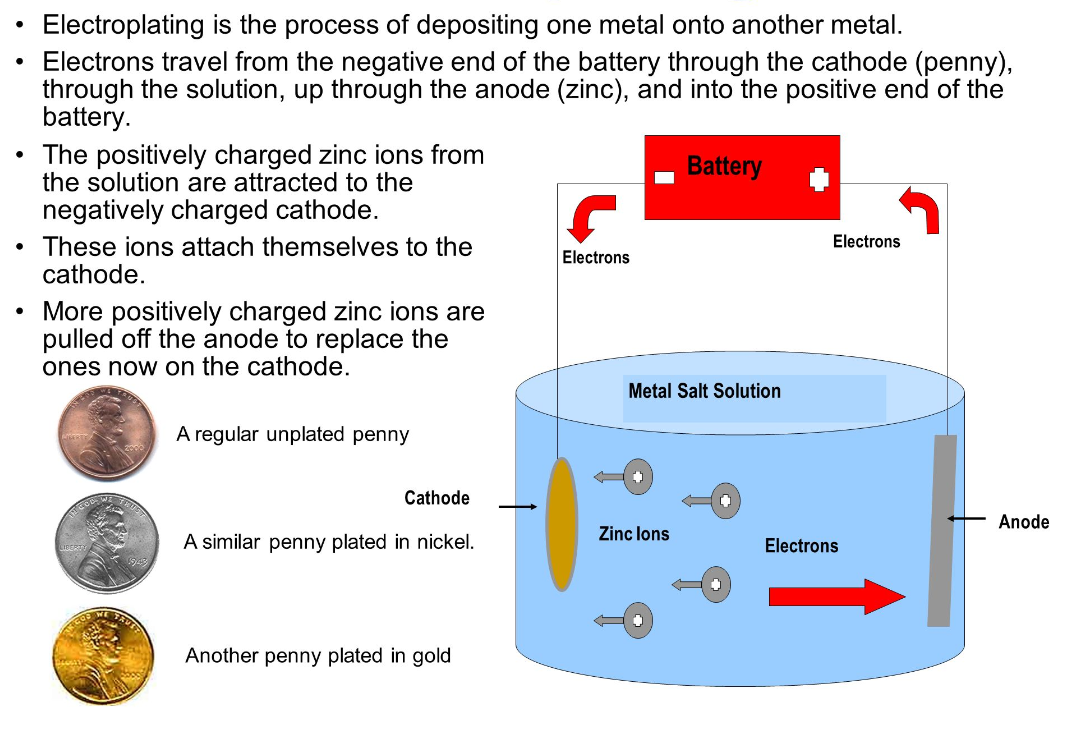
Electroplating is basically the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or for decorative purposes.
The process uses an electric current to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode. TOP Prototype company is actively involved in electroplating processes. Electroplating is often applied in the electrical oxidation of anions on a solid substrate like the formation of silver chloride on silver wire to form silver chloride electrodes.
Electroplating is majorly applied to modify the surface features of an object (e.g corrosion protection, lubricity, abrasion), but the process can also be used to build thickness or make objects by electro forming.
History of Electroplating

Boris Jacobi developed electroplating, electrotyping, and galvanoplastic sculpture in Russia. It was once theorized that the first electroplating was done in the Parthian Empire era.
Wilhelm König in the 1930s observed fine silver objects from ancient Iraq plated with thin gold layers and speculated they were electroplated, referring to a possible Parthian battery. However, this has been debunked and modern archaeologists agree the objects were fire-gilded with mercury.
Electroplating was actually invented by Italian chemist Luigi Valentino Brugnatelli in 1805. He used Alessandro Volta’s voltaic pile to facilitate the first electrodeposition. Brugnatelli’s inventions were suppressed and it wasn’t until 1839 that scientists in Britain and Russia independently devised metal-deposition processes similar to his for copper electroplating of printing press plates.

- 1805: Italian chemist Luigi Valentino Brugnatelli invented electroplating using Alessandro Volta’s voltaic pile. However, his inventions were suppressed and did not become used in general industry for the next thirty years.
- 1839: Scientists in Britain and Russia independently devised metal-deposition processes similar to Brugnatelli’s for the copper electroplating of printing press plates.
- 1840: George and Henry Elkington were awarded the first patents for electroplating and founded the electroplating industry in Birmingham, England.
- 1850s: Commercial electroplating of nickel, brass, tin, and zinc had been developed.
- 1876: The Norddeutsche Affinerie in Hamburg was the first modern electroplating plant starting production.
- Late 19th century: The plating industry grew with the development of electric generators.
World War eras and growing aviation industry: This led to further developments and refinements, including processes like hard chromium plating and sulfamate nickel plating. Plating equipment evolved from manually-operated wooden tanks to automated equipment.
At an unspecified time (but likely in the 20th century): Richard Feynman’s first project was to develop technology for electroplating metal onto plastic. In Russia at an unspecified time but likely before some of the other events: Boris Jacobi rediscovered galvanoplastics and developed electrotyping and galvanoplastic sculpture.
Galvanoplastics became fashionable in Russia with contributions from inventor Peter Bagration, scientist Heinrich Lenz, and author Vladimir Odoyevsky. Notable examples include the gigantic galvanoplastic sculptures of St. Isaac’s Cathedral in Saint Petersburg and the gold-electroplated dome of the Cathedral of Christ the Saviour in Moscow.
Types of Electroplating

Nickel Plating
Nickel plating provides a hard, durable surface that is resistant to corrosion. It also offers good wear resistance and can improve the appearance of the object.
Applications: Commonly used in the automotive industry for plating parts such as bumpers and trim. Also used in plumbing fixtures and hardware. Nickel plating can be done in different finishes, such as bright nickel (highly reflective) or satin nickel (a softer, matte look).
Chrome Plating
Chromium is a hard metal that resists scratches and corrosion. Chrome plating is often used for decorative purposes as well as to provide protection to underlying metals.
Applications: Widely used in the automotive industry for plating exterior parts like wheels and exhaust tips. Also found on bathroom fixtures and some kitchen appliances. Hard chrome plating is used in industrial applications for parts that require high wear resistance, such as hydraulic cylinders.
Gold Plating
Gold plating can enhance the appearance of an object and give it a luxurious look. It also provides some corrosion resistance.
Applications: Used in jewelry making to give a high-end finish. Also found on electrical connectors to ensure good conductivity and prevent corrosion. In some high-end electronics and luxury goods, gold plating is used for its aesthetic appeal.
Zinc Plating
Zinc plating is known for its excellent corrosion resistance, especially in outdoor or harsh environments.
Applications: Commonly used to protect steel parts from rust and corrosion. Found on items like nuts, bolts, and metal hardware. Zinc plating can be done by different methods, such as hot-dip galvanizing or electrogalvanizing.
Copper Plating
Copper is a good conductor of electricity and heat. Copper plating can improve electrical conductivity, enhance heat transfer, and provide a decorative finish.
Applications: Used in the electronics industry for printed circuit boards and electrical connectors. Also used in decorative applications such as statues and ornamental items.
How does Electroplating Work?

Electroplating works through a series of steps.
- First, the object to be plated is cleaned and prepared to ensure a good surface for the plating to adhere to. This often involves degreasing and sometimes etching.
- Next, the object is placed in an electrolyte solution. The electrolyte is a liquid that contains ions of the metal to be plated. For example, if gold plating is desired, the electrolyte will contain gold ions.
An anode, which is made of the metal to be plated, is also placed in the electrolyte. When a direct electric current is passed through the solution, a chemical reaction occurs.
The positive ions of the plating metal in the electrolyte are attracted to the negatively charged object (cathode). As they reach the cathode, they gain electrons and are deposited onto the surface of the object, forming a thin layer of metal.
The thickness of the plating can be controlled by factors such as the duration of the plating process, the strength of the electric current, and the composition of the electrolyte.
This process allows for a uniform and controlled deposition of metal onto the object, providing enhanced appearance, corrosion resistance, or other desired properties.

Advantages of Electroplating:
Enhanced Appearance
- Electroplating can give a product a shiny, lustrous finish. This makes it highly attractive and can increase the aesthetic value of items such as jewelry, hardware, and automotive parts.
- It can also create a wide range of colors and finishes, allowing for customization and meeting different design requirements.
Corrosion Resistance
- The deposited metal layer can protect the underlying substrate from corrosion. For example, zinc plating on steel provides a barrier that prevents moisture and oxygen from reaching the steel surface, significantly increasing the lifespan of the part.
- This is crucial in applications where the product will be exposed to harsh environments, such as marine or industrial settings.
Improved Wear Resistance
- The hard metal coatings obtained through electroplating can withstand abrasion and wear. Hard chrome plating is often used on parts that experience friction, such as hydraulic cylinders and machine tools.
- This reduces the need for frequent replacement of parts and lowers maintenance costs.
Enhanced Electrical Conductivity
- In some cases, electroplating with metals like copper or gold can improve the electrical conductivity of a surface. This is essential in the electronics industry for components like connectors and printed circuit boards.